CLOCKS FOR STUDYING TEMPORAL LAWS OF ANIMAL DEVELOPMENT
T. A. Dettlaff
The study of time as a natural science and philosophical category has a long history of more than 25 centuries (Podol'nyi 1988). Nevertheless, defining time in units that are suitable for quantitative accounting and reflect qualitative specificity of a studied phenomenon, which aid in identifying temporal principles, remains an actual and difficult task for many sciences up till now. Among the numerous definitions of time (Podol'nyi 1988) there was one adopted by Vernadskii (1988) that time is duration (continuation). This definition, in my opinion, is entirely adequate for sciences studying the development of animals. It conveys the primary properties of time: its continuity, irreversibility, and duration.
It is much more difficult to find an answer to the question of methods for measuring or gauging time. Units of astronomical time, widely used for this purpose (days, hours, minutes, and seconds), provide very limited information, useful in each case only for the given species of organisms and for the given specific conditions (see Dettlaff 1986).
A measure of time that would permit forecasting developmental time and provide descriptions of developmental time comparable under various conditions and for various animal species, and also identify the temporal principles of animals' development, has been the subject of searches by a large array of investigations carried out primarily by reseachers at the D. P. Filatov Laboratory for Experimental Embryology, and the N.K.Koltzov Institute for Developmental Biology (see Dettlaff and Dettlaff 1960, 1961, 1982; Dettlaff 1977; Ignatieva 1979; Dettlaff et al. 1987). This article will briefly lay out the major results of these investigations.
1. Biological measure of time
Fulfilling the set tasks required to overcome enormous difficulties related to the fact that development time for animals depends on temperature, and that each species has its own hereditary dependence between developmental rate and temperature. An additional difficulty was created by the widespread notion (see ![]() Error! Bookmark not defined.dek 1935; Hayes 1943; Svetlov 1978) whereby the developmental rate of both rudiments of various organs and of entire embryos at various stages depends on temperature in different ways. We were able to move toward solving these tasks only after establishing (Dettlaff 1953; Dettlaff and Dettlaff 1960, 1961) and repeatedly confirming (Dettlaff 1977; Ignatieva 1979; Dettlaff et al. 1987) the error of this concept, and showing that in the zone of optimal temperatures the duration of various organogeneses and various developmental periods in embryos of poikilothermic animals changes proportionally with each other.
Error! Bookmark not defined.dek 1935; Hayes 1943; Svetlov 1978) whereby the developmental rate of both rudiments of various organs and of entire embryos at various stages depends on temperature in different ways. We were able to move toward solving these tasks only after establishing (Dettlaff 1953; Dettlaff and Dettlaff 1960, 1961) and repeatedly confirming (Dettlaff 1977; Ignatieva 1979; Dettlaff et al. 1987) the error of this concept, and showing that in the zone of optimal temperatures the duration of various organogeneses and various developmental periods in embryos of poikilothermic animals changes proportionally with each other.
Let us cite here some of the initial data on which this conclusion is based. Figure 1 presents plots expressing the dependence of time of the onset of seven consecutive developmental stages in sturgeon's embryos on temperature (in hours) after insemination. Figure 2 presents the same data, but recalculated in the number of various developmental periods: a number of time periods between the first appearance on the egg surface of the first and second cleavage furrows (![]() ); the number of periods from insemination to onset of gastrulation (tn/tI); and in shares of the total period of embryonic development - from insemination to hatching of the advanced embryos from the egg envelopes (tn/tVII 100). Figure 3 presents the same empirical data plotted in logarithmic coordinates. Comparing Figures 1 and 2, it is clear that in the transition from describing time in hours to relative description of developmental time in a number of developmental periods taken as time units in the zone of optimal temperatures, the curves align and run more or less in parallel with the x-axis, i.e., the duration of various developmental periods in embryos following a change in temperature changes proportionally. The parallel and equidistant location of curves in Figure 3 are evidence of this.
); the number of periods from insemination to onset of gastrulation (tn/tI); and in shares of the total period of embryonic development - from insemination to hatching of the advanced embryos from the egg envelopes (tn/tVII 100). Figure 3 presents the same empirical data plotted in logarithmic coordinates. Comparing Figures 1 and 2, it is clear that in the transition from describing time in hours to relative description of developmental time in a number of developmental periods taken as time units in the zone of optimal temperatures, the curves align and run more or less in parallel with the x-axis, i.e., the duration of various developmental periods in embryos following a change in temperature changes proportionally. The parallel and equidistant location of curves in Figure 3 are evidence of this.
On the basis of these and similar data (Dettlaff and Dettlaff 1960, 1961; Dettlaff 1977), we conclude that for measuring the duration of any period of embryonic development, designated ![]() , the duration at the same temperature of some period tX taken as a unit of time may be used as a measure of time, i.e., may use a relative or dimensionless characteristics of development duration tn/tX. The quotient of dividing tn by tX shows how many times tX is greater (or smaller) than tn at any optimal temperature. Such a method for determining developmental time allowed us to "liberate" time from its dependence on temperature, since the value tn/tX is identical at various temperatures within the zone of optimal temperatures. It also liberated time from species specific dependence of developmental rate on temperature, since it is identical for tn and tX. Finally, it liberated us from astronomical units of time, which contract.
, the duration at the same temperature of some period tX taken as a unit of time may be used as a measure of time, i.e., may use a relative or dimensionless characteristics of development duration tn/tX. The quotient of dividing tn by tX shows how many times tX is greater (or smaller) than tn at any optimal temperature. Such a method for determining developmental time allowed us to "liberate" time from its dependence on temperature, since the value tn/tX is identical at various temperatures within the zone of optimal temperatures. It also liberated time from species specific dependence of developmental rate on temperature, since it is identical for tn and tX. Finally, it liberated us from astronomical units of time, which contract.
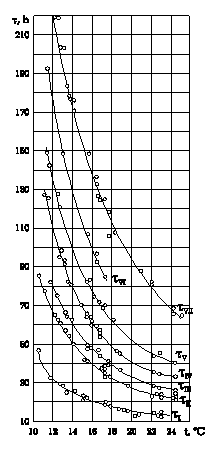
Fig.1.Duration of development in sturgeon's embryos from insemination to onset of gastrulation (tI), to end of gastrulation (tII), to closure of nerve folds (tIII), to closure of lateral plates in region of rudimentary heart (tIV), to onset of heartbeat (tV), to stage when the tail end touch head (tVI), to hatching (tVII). Abscissa: temperatureoC; ordinate: time, h.

Fig.2.Duration of various developmental periods (tI,...,tVII) in sturgeon, expressed in dimensionless units:a)as the number of periods of one mitotic cycle in first cleavage divisions (tn/t0 ); b)as the number of periods of division (tn/tI);c)as the percent of total duration of development (from insemination to onset of hatching) (tn/tVII·100). Abscissa: temperature, oC; ordinate:duration of various developmental periods, expressed in various relative units (a,b,c).

Fig.3. Dependence of logarithm for duration of developmental periods ![]() on logarithm for temperature. Abscissa: temperature, ° C; ordinate: time, h.
on logarithm for temperature. Abscissa: temperature, ° C; ordinate: time, h.

Fig. 4. The value of (duration of τ0 one mitotic cycle during the period of synchronous cleavage divisions) in sturgeon.
Various clearly defined developmental periods may serve as a measure of time for each animal species. However, in order to compare developmental time of various animals, and to identify temporal principles common to them, it is important that the developmental period taken as a measure of time be equivalent in various animals. It turned out that hardly any such equivalent developmental periods exist as a result of the widespread phenomenon of heterochrony: in various animal species: fertilization occurs at various meiotic stages, gastrulation occurs at various cleavage stages, hatching of embryos occurs at various developmental stages, and so forth. One exception is the mitotic cycle during the period of synchronous cleavage division, which occurs during the development of the majority of poikilothermic animals: worms, molluscs, arthropods, echinoderms, fish, amphibians, and also birds (see Dettlaff 1977; Rott 1980, 1987).
The mitotic cycle during synchronous cleavage is characterized by a series of common traits: minimal duration, virtually identical relative duration of analogous mitotic phases, and absence of the G1 and G2 phases typical for the complete cycle. Granular nucleolus is absent in the nuclei, and thus no RNA synthesis occurs. The cytoplasm contains a large pool of precursors of macromolecular syntheses. The presence of all these traits in various animal species made it possible to select the duration of one mitotic cycle during the period of synchronous cleavage divisions, which we denote with the symbol ![]() (The proposed unit cannot be used for mammals or certain animal species with mosaic-type cleavage, in which the period of synchronous cleavage divisions is absent) as a measure of time. Several investigators follow Neyfakh's (1961) suggestion to call it a detlaf. The duration of t0 is equal to the interval between the appearance of analogous mitotic phases of two consecutive cleavage divisions.
(The proposed unit cannot be used for mammals or certain animal species with mosaic-type cleavage, in which the period of synchronous cleavage divisions is absent) as a measure of time. Several investigators follow Neyfakh's (1961) suggestion to call it a detlaf. The duration of t0 is equal to the interval between the appearance of analogous mitotic phases of two consecutive cleavage divisions.
In order to use t0 as a measure of time, its value in minutes in the zone of spawning temperatures must be determined for each animal species, and a curve must be plotted for dependence of t0 on temperature, similar to that in Fig. 4. Duration of the mitotic cycle at varying temperature was determined from the duration of the cytokinesis cycle at the same temperature. Cytological study established preliminarily that the mitotic cycle in sturgeons (Ginsburg 1959) and amphibians (Skoblina 1965; Rudneva 1972; Rott 1973) is equal to the interval between the first appearance on the egg surface of the 1st and 2nd cleavage furrows, and in teleostean fishes (see Ignatieva 1979) equals half the interval between the appearance of the 2nd and 4th cleavage furrows (t01) or one-fifth of the interval between insemination and the appearance of the IV cleavage furrow (t02). Curves of the dependence of t0 on temperature were plotted for four sturgeon species (Dettlaff et al. 1993), eight teleostean species (Ignatieva and Kostomarova 1966; Kostomarova and Ignatieva 1968; Ignatieva 1979), ten amphibian species (see Dettlaff 1986; Mazin and Dettlaff 1985), and one starfish species (Davydov et al. 1989). In addition, the value of the cytokinesis cycle in the first cleavage divisions (hypothetically equal to the mitotic cycle) was determined at several temperatures in two sea urchin species (Buznikov and Podmarev 1990) and in one gastropod species (Meshcheryakov 1990).
These data support the statement that in the zone of optimal temperatures, the duration of various developmental periods in each species changes proportionally. It was shown further that the proportionality of changing duration of various developmental periods persists not only during the embryonic stages, but also during the prelarval stages as well as during the period of maturation of oocytes in the ovaries and in vitro in a physiological solution (see Dettlaff et al. 1993). This means that a change in temperature proportionally changes the duration of processes having the most diverse nature which occurs at various levels of organisation: intracellular (molecular and ultrastructural), cellular (with division of cells and their differentiation), and at the level of morphogenetic movements and the processes of induction and organogenesis. Thus we propose that it occurs at some higher levels of regulation common to the most diverse phenomena of life and, possibly, involved in the housekeeping function of genes. We mention this in order to direct the attention of investigators to the study of this just arisen question. According to L.N.Lyubinskaya (Dettlaff and Lyubinskaya 1987), the proportionality of changes in duration of such diverse processes with the temperature may be described by using the so-called affine geometry.
The proportionality of changes in duration of various processes and various developmental periods following temperature changes within the limits of optimal for each species of poikilothermic organisms is of great significance. It would not be an exaggeration to say that without this ability, poikilothermic organisms generally could not exist as the environmental conditions change. If various components of the set of processes at any developmental stage changed asynchronously, that would lead to the disturbance of normal development and, at later stages, of abnormal functioning of the organism. It is no mistake that one of the first reactions of an embryo when approaching the limits of optimal tempertaures is desynchronization of individual developmental processes. At first, it is usually small, and is compensated for by the organism's regulational reserve (Shmalhausen 1938, 1964), but with large deviations in temperature it goes beyond the limits of regulated changes, and this leads to abnormal development and death of the organism.
Thus, the proportionality of changes in duration of various periods and developmental processes following a temperature change within the limits of the biological optimum is a necessary condition both for integrity of the developing organism, and for its adaptation to changing environmental conditions (Dettlaff 1981, 1985). It is a sure foundation for wide utilization of the method of relative, dimensionless characteristics developmental duration, and for introducing the study of developmental biology of poikilothermic animals the criterion of biological time comparable for all animals under temperature conditions that are variable, but optimal for each species.
There is another important fact that cannot be forgotten, i.e., the relative measure of time may be used only under conditions in which the studied developmental stages retain their proportionality of the change in values of tn and t0. Meanwhile, the limits of optimal temperatures may shift as development progresses (for example, see Zotin et al. 1989). For later stages, they generally shift toward somewhat higher temperatures, of which the extremes may damage the early embryos. As a result, the amplitude of temperatures at which the proportionality of changes in tn and t0 persists may narrow during the process of development. This relates particularly to the slowly developing species, those that reproduce in autumn, and prelarvae of spring-spawning fish.
2. Application of the method of relative dimensionless characteristic of developmental duration for forecasting values of t n at varying temperature
We begin by forecasting the timing of various stages and the duration of various developmental periods. Remember that since tn and t0 change proportionally in the zone of optimal temperatures, the quotient of dividing tn by t0 defined in minutes at the same temperature optimal for them, is the value of the constant for the entire zone optimal of temperatures. Therefore, after determining tn/t0 at one temperature and plotting the curve for the dependence of t0 on temperature, we can mathematically determine the value of tn in minutes for any temperature. To this end, the quotient of dividing tn by t0 must be multiplied by the value of t0 taken from the curve. Such a method for determining tn reduces the investigator's labor, freeing him from the need to obtain new empirical data for each temperature. This makes it possible to calculate the time for processes not easily accessible for direct observation (for example, those occurring in ovarian oocytes within the body of the female or in nuclei of dividing cells). In addition, the results obtained with the method of relative characteristic of developmental duration often make it possible to detect mistakes in empirical observations.
Our proposed method has been widely adopted facilitate, e. g., in the books "Subjects Developmental Biology" (in Russian) (1975) and "Animal Species for Developmental Studies" vol. 1 (1990), and vol. 2 (1991). Data obtained for several animal species widely use in experimental studies and fisheries has been published. They contained tables of normal development, both from the literature and specially compiled for these editions. Along with "Number of stages" and "Time of their onset in minutes and hours", there is "Time in number of t0". Curves of t0 dependence on temperature are presented for all these animals.
The method of relative characteristic of developmental time was used as well for all four species of sturgeon to compile charts allowing us to calculate, at varying temperature, the time for injecting into the breeders a pituitary suspension to stimulate their maturation so that females mature at a time convenient for fisheries managers. Other compiled charts make it possible to determine the interval of time during which injected females should be examined in order to obtain good quality eggs for breeding. In addition, charts were compiled to allow forecasting of the time of onset of stages for recommended evaluation of the quality of insemination and embryonic development. Substantial retardation in maturation of females or in development of embryos and prelarvae, as compared with forecasts, indicates insufficiently favorable environmental conditions for them. Introducing forecasting of time of females' maturation and egg development not only made it possible to increase efficiency of sturgeons breeding, but also eased the labor of fish breeders (see Dettlaff et al. 1993).
Of no less significance is the possibility for forecasting the developmental time of experimental animals in science for planning experiments. Specifically, this makes it possible to conduct experiments with varying kinds of effects on various animals and at various temperatures more correctly at times biologically equivalent for them, as measured by an equal number or equal share of t0. Unfortunately, this possibility is still not appreciated by the majority of experimenters, and is used insufficiently in laboratory investigations.
3. Temporal patterns of development in poikilothermic animals
We will discuss briefly here the question of which temporal patterns of development have been identified with the method of relative dimensionless characteristic of developmental duration. The issue here will be the time laid out in the genetic program for development of each animal species; our method liberates us from the need to consider this time's dependence on temperature, and on the hereditary dependence of developmental rate on temperature. This program involves determination of the time for the embryo to progress from one developmental period to another, and the rate with which the embryo passes through successive periods and individual morphogenetic processes. Using the relative characteristic of developmental duration allows us to compare them with different animals. To compare the developmental rate in various animals, we introduced a criterion of relative developmental rate (RDR), equal to the quotient of dividing (tn/t0)1 of one species by (tn/t0)2 of another (Dettlaff 1986)
![]() .
.
In cases where the transition from one developmental period to another occurs at the same developmental stage in compared animals species but with a smaller or larger number of t0, and the RDR in compared species is greater or less than unity, we speak of their different development rates. In those cases where the transition from one developmental period to another in compared species is "programmed" at various developmental stages, shortening or lengthening of the previous period occurs by means of earlier or later onset of the subsequent period. In this case we are dealing with heterochronies, which should be distinguished from a change in developmental rate. To identify heterochronies, it is sufficient to conduct a thorough comparative morphological investigations, and using the relative characteristic developmental duration permits dating them as number of t0, which is comparable for various animals and under various conditions.
The most common of the established patterns is conservatism of temporal organization of early developmental processes in various animals. Duration of analogous phases of the mitotic cycle during the period of synchronous cleavage divisions in such remote animals as sea urchins and various species of sturgeons, teleostean fish, and amphibians is measured by identical or nearly identical shares of t0, i. e., it lasts an identical biological time in various animals (while the duration of mitotic phases, measured in minutes, may differ by ten times) (Dettlaff 1963, 1977; Skoblina 1965; Rudneva 1972; Ignatieva 1973, 1979; Dettlaff et al. 1987). An identical number of ![]() is also found in the duration of periods of insemination and synchronous cleavage divisions in embryos of telostean fish belonging to various families (Ignatieva 1973; Rott 1987).
is also found in the duration of periods of insemination and synchronous cleavage divisions in embryos of telostean fish belonging to various families (Ignatieva 1973; Rott 1987).
Unlike the very early developmental stages, the duration of analogous developmental periods is measured by an identical number of t0 only in closely related species, but sometimes also in animals belonging to various genera and even families, often geographically very remote from one another. This was found in four representatives of sturgeons belonging to two different genera, two salmonid species, two whitefish species, various frog species, and various representatives of urodelean amphibians (Dettlaff et al. 1987).
The constant (Dettlaff et al. 1993) of relative developmental rate (RDR = 1) was found to be retained in related animal species over a varying amount of time. In embryos of sevruga, sturgeon, beluga, and sterlet, RDR = 1 until the middle of the period of embryonic development, while subsequent developmental periods of the beluga occur at decreasing number of t0, i. e., they accelerate (Igumnova1975, 1985). Embryos and prelarvae of sturgeon and sevruga develop with identical relative rate (RDR = 1) to the stage when gill respiration begins, but the subsequent period, from the onset of gill respiration to the beginning of active food capture, proceeds in sturgeon prelarvae at a smaller number of ![]() than in sevruga prelarvae (see Dettlaff 1986, Dettlaff et al. 1993). It still has not been established when RDR begins to deviate from unit in various frog species and in various representatives of urodelean amphibians. Judging from tables of normal development, embryos of axolotl and spanish newt, which belong to different families of Urodela, proceed through analogous developmental periods at identical t0 number (RDR = 1) up to the stage of liberation from their egg envelopes. In teleostean, it has been established that embryos of two trout species develop with identical relative rate up to the stage of 10 somite pairs; the same is true of two whitefish species (peled and chir their development has not been followed further) (Ignatieva 1979).
than in sevruga prelarvae (see Dettlaff 1986, Dettlaff et al. 1993). It still has not been established when RDR begins to deviate from unit in various frog species and in various representatives of urodelean amphibians. Judging from tables of normal development, embryos of axolotl and spanish newt, which belong to different families of Urodela, proceed through analogous developmental periods at identical t0 number (RDR = 1) up to the stage of liberation from their egg envelopes. In teleostean, it has been established that embryos of two trout species develop with identical relative rate up to the stage of 10 somite pairs; the same is true of two whitefish species (peled and chir their development has not been followed further) (Ignatieva 1979).
4. Heterochrony
Comparing development of embryos from various frog species with that of newt and axolotl embryos (Detlaff 1954) showed that even at the early stages there were differences in the genetic program of temporal parameters of their development displaying a heterochronic nature: Gastrulation in newt and axolotl embryos begins at earlier stages of cleavage with a smaller t0 number than in frogs.
Similar temporal patterns of development have also been found in teleostean fish (Ignatieva 1979; Dettlaff et al. 1987). After the periods of fertilization and synchronous cleavage divisions, the embryos of various families pass from blastulation to gastrulation over time measured by different numbers of t0. Heterochrony in early development of embryos from various families of teleostean fish appears at various stages of development, like in embryos of urodelean and anuran amphibians, specifically at those stages when the transition to the subsequent developmental period requires switching on the gene activity (Neifakh 1961; Ignatieva 1979). It is hoped that further investigations will expand our knowledge on these questions.
5. Age of embryos and its significance for differentiation
Detection of heterochronics at early developmental stages is of great significance for correctly estimating the age of embryos. This is because the age of embryos of poikilothermic animals cannot be determined in astronomical units of time, since at various temperatures it varies at a given level of differentiation. Therefore, for lack of other criteria, age is often equated to the concept of stage, and embryos of various species at one developmental stage are considered to be of similar age. Moreover, as we have repeatedly written (Dettlaff 1956, 1964), this is incorrect since a shift in the onset of gastrulation to earlier or later stage of cleavage divisions results in embryos of representatives from various systematic groups at similar stages of gastrulation and neurulation consisting of cells of various cellular generations and containing varying numbers of cells. Only defining the embryo's age as the time from insemination, expressed as the number of ![]() , makes it possible to compare the age of embryos of various animals, at various developmental stages. The possibility of estimating and comparing age of embryos of various animals and precisely determining the time of their development made it possible for the first time to introduce the parameter of time into comparative investigations of the causal mechanisms of individual development with which modern developmental biology is concerned. For example, Ignatieva (1979) used the method of relative description of changes in spatial and temporal rations between studied presumptive rudiments during gastrulation in her comparative study of the dynamics of morphogenetic movements during this period in sturgeon and axolotl embryos (she expressed time in the number of t0 and shares of the gastrulation period, and sizes in angular degrees and radians). This make it possible to establish how harmonious combination of parts was accomplished and similar construction of embryos at the late gastrula stage arose when there were initial differences in the topography and morphogenetic properties of presumptive rudiments in compared species during gastrulation.
, makes it possible to compare the age of embryos of various animals, at various developmental stages. The possibility of estimating and comparing age of embryos of various animals and precisely determining the time of their development made it possible for the first time to introduce the parameter of time into comparative investigations of the causal mechanisms of individual development with which modern developmental biology is concerned. For example, Ignatieva (1979) used the method of relative description of changes in spatial and temporal rations between studied presumptive rudiments during gastrulation in her comparative study of the dynamics of morphogenetic movements during this period in sturgeon and axolotl embryos (she expressed time in the number of t0 and shares of the gastrulation period, and sizes in angular degrees and radians). This make it possible to establish how harmonious combination of parts was accomplished and similar construction of embryos at the late gastrula stage arose when there were initial differences in the topography and morphogenetic properties of presumptive rudiments in compared species during gastrulation.
Using the concept of embryonic age has great significance in comparative studies of determination, i.e., the acquisition by an embryo's cells of new properties ensuring at least in part their development toward their final goal (formation of a particular organ or organ part). Studying this process is one of the central tasks of experimental embryology and, more generally, developmental biology. It was shown that determinations of presumptive material of analogous organ rudiments (placodes of sensory organs, nervous lamella, and others) in various urodelean and anuran amphibian species arised at various developmental stages and were expressed differently at identical stages (Filatov 1943). Moreover, it turned out that these differences correlate with differences in the age of embryos at the analogous stages of development (Ginsburg 1950, 1989; Dettlaff 1956, 1964; Dettlaff et al. 1987). The correlation of the level of determination with the age of embryos gave rase to the question of the morphogenetical role of the time factor. There has previously been reason to suggest the existence of such a role; for example, it was shown that when ectoderm from an embryo was cultivated in saline, its response to inductive actions gradually changed (Holtfreter 1938). However, at that time this question still could not be posed in full measure, specifically because of the absence of strict criteria for estimating the age of embryos, as well as with the absence of experimental approaches to its analysis (see Dettlaff et al. 1987; Ginsburg 1989).
In conclusion, it should be said that the problem of biological time is a very important and multifaceted for developmental biology. The method of relative, dimensionless characteristic of developmental duration opens up wide possibility for its study, and we should hope that these possibilities will be used in full measure.
REFERENCES
![]() HRБDEK J. (1935). Temperature and Living Matter. In: Protoplasma Monographien 8. Berlin.
HRБDEK J. (1935). Temperature and Living Matter. In: Protoplasma Monographien 8. Berlin.
BUZNIKOV G. A. and PODMAREV V. I. (1990). Sea urchins. Strongylocentrotus drobachiensis, S. nudus, S. intermedius. Animal Species for Developmental Studies.V.1. Invertebrates. Pp.253 - 286.
DAVYDOV P. V., SHUBRAVYI O. I. and VASSETZKY S. G. (1990). The Starfish Asterina pectinifera (Muller et Froschel, 1842). In: Ibid. V.1. Pp.287 - 311.
DAVYDOV P.V., SHUBRAVYI O. I. and VASSETZKY S. G. (1989). Duration of the mitotic cycle in the synchronous cleavage period (![]() in the Starfish Asterina pectinifera (Muller et Froschel). Sov. J. Devel. Biol.V.20. N.1. Pp.28-32.
in the Starfish Asterina pectinifera (Muller et Froschel). Sov. J. Devel. Biol.V.20. N.1. Pp.28-32.
DETTLAFF T. A. (1953). Dependence of rate of cleavage in sturgeon eggs on temperature. Dokl. Acad. Nauk SSSR. V.91. Pp.695-698.
DETTLAFF T. A. (1956). Species differences in formative properties of embryonic material and shift in gastrulation relative to cleavage stages. Significance of correlation of developmental stages and cellular generations. Dokl. Akad. Nauk SSSR. V.111. Pp.1149-1152.
DETTLAFF T. A. (1963). Mytotic dynamics of the first cleavage division in the eggs of Sturgeon (at various temperatures) and of trout. Exp. Cell Res. V.29. Pp.499-503.
DETTLAFF T. A. (1964). Cell divisions, duration of interkinetic states, and differentiation in early stages of embrionic development. Adv. Morphog. V.3. Pp.323-362.
DETTLAFF T. A. (1965). Duration of interkinetic states of cells, cell divisions and differentiation. In: Cellular Differentiation and Inductive Mechanisms. Pp.147-159.
DETTLAFF T. A. (Ed.) (1975): Subjects in Developmental Biology. Moscow (in Russian).
DETTLAFF T. A. (1977). Some thermal-temporal patterns of embryogenesis in poikilothermic animals. In: Problems in Experimental Biology. Belyaev D. K. (Ed.). Pp.269-289. Moscow (in Russian).
DETTLAFF T. A. (1981). Adaptation of poikilothermic animals to development under conditions of fluctuating temperatures and the problem of the integrity of the developing organism. Sov. J. Dev. Biol. V. 12. Pp.139 -151.
DETTLAFF T. A. (1985). On general principles of the organism integrity in ontogenesis. Zh. Obshch. Biol. V.46. Pp.147- 152 (in Russian).
DETTLAFF T. A. (1986a). Developmental velocity in poikilothermic animals. Zh. Obshch. Biol. V.47. Pp.163-172 (in Russian).
DETTLAFF T. A. (1986b). The rate of development in poikilothermal animals calculated in astronomical and relative time units. J. Term. Biol. V.11. Pp.1-7.
DETTLAFF T. A. (1991). Temperature and timing in developmental biology. Animal Species for Developmental Studies. T. A. Dettlaff and S. G. Vassetzky. (Eds.).V.2. Pp.1-14.
DETTLAFF T. A. and DETTLAFF A. A. (1960). Dimensionless characteristics of developmental duration in embryology. Dokl. Akad. Nauk SSSR. V.136. Pp.199-202.
DETTLAFF T. A. and DETTLAFF A. A. (1961). Relative dimensionless characteristics of the development duration in embryology. Arch. Biol. (Liйge). 72. Pp.1-16.
DETTLAFF T. A. and DETTLAFF A. A. (1982). Dimensionless criteria as a method for quantitative description of animal development. In: Mathematical Developmental Biology. Zotin A. I. and Presnov E. V. (Eds.). Moscow. Pp.25-39 (in Russian).
DETTLAFF T. A., GINSBURG A. S. and SHMALHAUSEN O. I. (1993). Sturgeon Fishes. Developmental Biology and Aquaculture. Berlin.
DETTLAFF T. A., IGNTATIEVA G. M. and VASSETZKY S. G. (1987). The Problem of Time in Developmental Biology. Its Study by the Use of Relative Characteristics of Development Duration. Sov. Sci. Rev. Ser. F. Physiol., Gen., Biol. 1. Pp.1-88.
DETTLAFF T. A. and LYUBINSKAYA L. N. (1987). On the question of certain mechanisms of self-organization. In: Self-propulsion Self-organization, Self-management. Summary of Reports of the Interinstitute Scientific Conference (in Russian).
DETTLAFF T. A. and VASSETZKY S. G. (Eds.) (1990). Animal Species for Developmental Studies. V.1. 1990. V. 2. 1991.
FILATOV D. P. (1943). Mechanics of Development as a method for studying certain questions of evolution. Zh. Obshch. Biol. Pp.28-64 (in Russian).
GINSBURG A. S. (1950). Species specifics of initial stages of development of the labyrinth in amphibians. Dokl. Akad. Nauk SSSR. V.73. Pp.229-232.
GINSBURG A. S. (1959). Fertilization in Sturgeons. 1 Union of gametes. Tsitologyia 1. Pp.510-526 (in Russian).
GINSBURG A. S. (1989). Determination of derivatives of ectoderm in various amphibian species. J. Dev. Biol. V.20. Pp.18-27.
HOLTFRETER J. (1938). Differenzierungspotenzen isolierter teile der urodelengastrula. W. Roux Arch. Entwicklungamech. Organismen.V.138. Pp.522-656.
HAYES F. R. (1949). The growth, general chemistry and temperature reactions of salmonid eggs. Q. Rev. Biol. V.24. Pp.281-308.
IGNATIEVA G. M. (1973). Relative duration of processes of karyo- and cytotomy during period of synchronous cleavage divisions in carp and pike at various temperatures. Ontogenez. V.3. Pp.17-21 (in Russian).
IGNATIEVA G. M. (1979). Early Embryogenesis of Fishes and Amphibians (Comparative Analysis of Temporal Principles of Development). Moscow (in Russian).
IGNATIEVA G. M. and KOSTOMAROVA A. A. (1966). Duration of mitotic cycle during period of synchronous cleavage divisions (![]() ) and its dependence on temperature in embryos of loach. Dokl. Acad. Nauk SSSR. V.168. Pp.122-124.
) and its dependence on temperature in embryos of loach. Dokl. Acad. Nauk SSSR. V.168. Pp.122-124.
IGUMNOVA L. V. (1975). Chronological patterns of embryonic development of the beluga. Sov. J. Dev. Biol.V. 6. Pp.38-43.
IGUMNOVA L. V. (1985). Temporal pattern of embryogenesis in the sterlet. Ontogenez. V.16. Pp.67-72.
KOSTOMAROVA A. A. and IGNATIEVA G. M. (1968). Correlation of processes of kario- and cytotomy during period of synchronous cleavage divisions in loach (Misgurnus fossilis L.). Dokl. Acad. Nauk SSSR. V.183. Pp.490-492.
MAZIN A. L. and DETTLAFF T. A. (1985). Dependence of duration of one mitotic cycle during period of synchronous cleavage divisions on temperature in four species of Rana in limits of optimal temperature for their reproduction and early development. Ontogenez. V.16. Pp.382-388.
MESHCHERYAKOV V. N. (1990). The Common Snail pond (Limnaea stagnalis). In: Animal Species for Developmental Studies. 1. Invertebrates. Pp.69-132.
NEIFAKH A. A. (1961). Comparative radiation investigation into morphogenetic functions of nuclei in development of animals. Zh. Obshch. Biol. V.22. Pp.42-57 (in Russian).
PODOLINYI R. (1988). Controversy over tralphadorts. Znanie-Sila. V.4. Pp.19-43 (in Russian).
ROTT N. N. (1973). Correlation between karyo- and cytokinesis during the first cell divisions in the axolotl (Ambystoma mexicanum Cope). Sov. J. Dev. Biol. V.4. Pp.175-177.
ROTT N. N. (1980). Cell divisions during pregastrulation period of development. Sov. J. Dev. Biol. V.11. Pp.1-17.
ROTT N. N. (1987). Cell Cycles in Early Development of Animals. Moscow (in Russian).
RUDNEVA T. B. (1972). Duration of karyomitosis and cell division in cleavage division in clawed frog Xenopus laevis. Sov. J. Dev. Biol. V.3. Pp.526-530.
SHMALHAUSEN I. I. (1938). The Organism as a Whole in Development and Evolution. Moscow (in Russian).
SHMALHAUSEN I. I. (1964). Regulation of Formative Processes in Individual Development. Moscow (in Russian).
SKOBLINA M. N. (1965). Dimensionless description of duration of mitotic phases of early cleavage divisions in axolotl. Dokl. Acad. Nauk SSSR. V.160. Pp.700-703.
SVETLOV P. G. (1978). Physiology (Mechanics) of Development. Leningrad (in Russian).
VERNADSKII V. I. (1988). Philosophical Thoughts of a Naturalist. Moscow (in Russian).
ZOTIN A. I., ALEKSEEVA T. A. and OZERNYUK N. D. (1989). Method for determining optimal developmental conditions according to total oxygen consumption. Sov. J. Dev. Biol. V.20. Pp.61-66.